Giúp mình với ạ, mình đang cần gấp :< mình cảm ơn ạaaa

H24
Những câu hỏi liên quan
Giúp mình với ạ mình đang cần gấp ạ,Mình cảm ơn

 Giúp mình với ạ, mình đang cần gấp,mình cảm ơn ạ!
Giúp mình với ạ, mình đang cần gấp,mình cảm ơn ạ!
Mình đang cần gấp ạ, giải giúp mình với ạ. Mình cảm ơn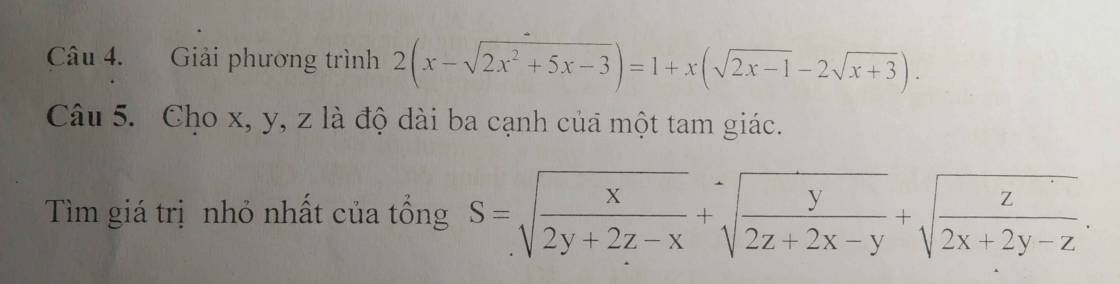
giúp mình với ạ mình đang cần gấp, cảm ơn ạ!!
1. How many kilos of potatoes would you like?
2. I went fishing but I didn't catch any fish.
3. Cook the chicken over low heat for 15 minutes before you serve it.
4. Can you tell me how to cook broken rice?
5. There are three cartons of milk in the fridge.
6. He has an egg but he hasn't got any bread.
7. Pho is one of the most popular dishes in Vietnam.
Đúng 3
Bình luận (0)
Giúp mình với ạ mình đang cần gấp ạ cảm ơn
1) \(\left(\dfrac{-13}{17}-\dfrac{31}{52}\right)-\left(\dfrac{73}{52}-\dfrac{13}{17}+\dfrac{5}{6}\right)-\dfrac{3}{4}\)
\(=\dfrac{-13}{17}-\dfrac{31}{52}-\dfrac{73}{52}+\dfrac{13}{17}-\dfrac{5}{6}-\dfrac{3}{4}\)
\(=\left(\dfrac{-13}{17}+\dfrac{13}{17}\right)-\left(\dfrac{31}{52}+\dfrac{73}{52}\right)-\left(\dfrac{5}{6}+\dfrac{3}{4}\right)\)
\(=0-2-\dfrac{19}{12}\)
\(=-2-\dfrac{19}{12}\)
\(=\dfrac{-43}{12}\)
Đúng 1
Bình luận (0)
2) \(\dfrac{1}{7}.\dfrac{1}{3}+\dfrac{1}{7}.\dfrac{1}{2}-\dfrac{1}{7}\)
\(=\dfrac{1}{7}\left(\dfrac{1}{3}+\dfrac{1}{2}-1\right)\)
\(=\dfrac{1}{7}.-\dfrac{1}{6}\)
\(=-\dfrac{1}{42}\)
Đúng 2
Bình luận (0)
3) \(\dfrac{13}{123}.\dfrac{1}{2}-\dfrac{1}{3}.\dfrac{13}{123}+\dfrac{1}{6}.\dfrac{110}{123}\)
\(=\dfrac{13}{123}.\left(\dfrac{1}{2}-\dfrac{1}{3}\right)+\dfrac{1}{6}.\dfrac{110}{123}\)
\(=\dfrac{13}{123}.\dfrac{1}{6}+\dfrac{1}{6}.\dfrac{110}{123}\)
\(=\dfrac{1}{6}\left(\dfrac{13}{123}+\dfrac{110}{123}\right)\)
\(=\dfrac{1}{6}.\dfrac{123}{123}\)
\(=\dfrac{1}{6}\)
Đúng 2
Bình luận (0)
Xem thêm câu trả lời
Giúp mình với ạ ! Mình đang cần gấp. Cảm ơn ạ !
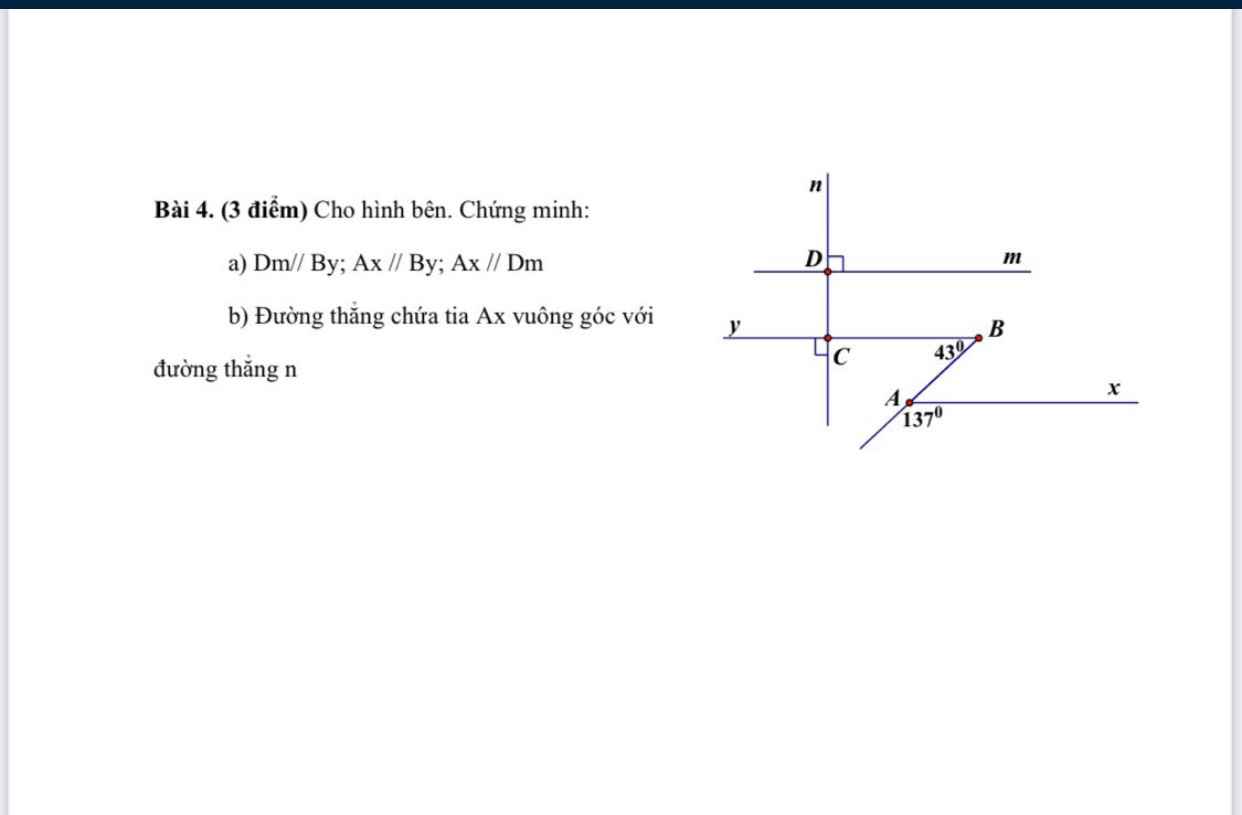
 Giúp mình với ạ mình đang cần gấp lắm :'(( mình cảm ơn ạ
Giúp mình với ạ mình đang cần gấp lắm :'(( mình cảm ơn ạ
Giúp mình với ạ mình đang cần gấp, mình xin cảm ơn trước ạ
PTHH: Al2O3+6HCl➝2AlCl3+3H2O(1)
a)nAl2O3=\(\dfrac{10,2}{102}\)=0,1(mol)
mHCl=\(\dfrac{5\%.219}{100\%}\)=10,95(g)
⇒nHCl=\(\dfrac{10,95}{36,5}\)=0,3(mol)
Xét tỉ lệ Al2O3:\(\dfrac{0,1}{1}\)=0,1
Xét tỉ lệ HCl:\(\dfrac{0,3}{6}\)=0,05
⇒HCl pứng hết,Al2O3 còn dư
Theo PTHH(1) ta có nAl2O3 pứng=\(\dfrac{nHCl}{6}\)=\(\dfrac{0,3}{6}\)=0,05(mol)
⇒nAl2O3 dư=nAl2O3ban đầu-nAl2O3 pứng=0,1-0,05=0,05(mol)
⇒mAl2O3 dư=0,05.102=5,1(g)
b) C%HCl=\(\dfrac{0,3.36,5}{219+10,2}\).100%=4,8%
nAlCl3=0,1(mol)
⇒C%AlCl3=\(\dfrac{0,1.136,5}{10,2+219}\).100%=6%
Đúng 1
Bình luận (0)
Giúp mình bài này với ạ mình đang cần gấp. Mình cảm ơn ạ



