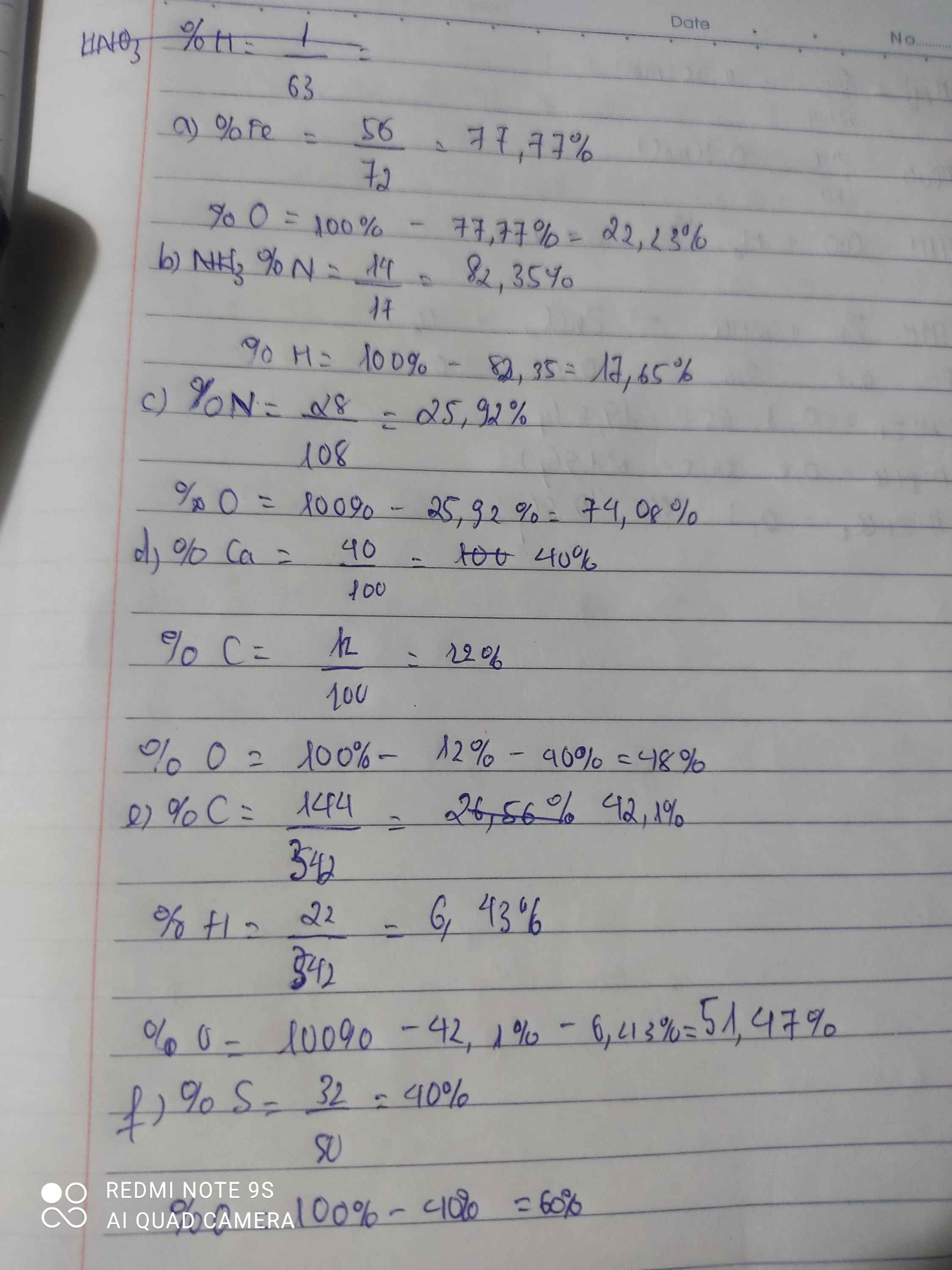a, \(\%m_{Fe}=\dfrac{56}{56+16}.100\%\approx77,78\%\)
\(\%m_O\approx22,22\%\)
b, \(\%m_N=\dfrac{14}{14+3.1}.100\%\approx82,35\%\)
\(\%m_H\approx17,65\%\)
c, \(\%m_N=\dfrac{14.2}{14.2+16.5}.100\%\approx25,93\%\)
\(\%m_O\approx74,07\%\)
d, \(\%m_{Ca}=\dfrac{40}{40+12+16.3}.100\%=40\%\)
\(\%m_C=\dfrac{12}{40+12+16.3}.100\%=12\%\)
\(\%m_O=48\%\)
e, \(\%m_C=\dfrac{12.12}{12.12+22+16.11}.100\%\approx42,11\%\)
\(\%m_H=\dfrac{22}{12.12+22+16.11}.100\%\approx6,43\%\)
\(\%m_O\approx51,46\%\)
f, \(\%m_S=\dfrac{32}{32+16.3}.100\%=40\%\)
\(\%m_O=60\%\)
Bạn tham khảo nhé!
a) \(M_{FeO}=56+16=72\left(\dfrac{g}{mol}\right)\)
\(\%Fe=\dfrac{56}{72}.100\%=77,7\%\)
\(\%O=100\%-77,7\%=22,3\%\)
Còn lại bạn làm như bài này nè
a)\(M_{FeO}=56+16=72đvC\)
\(\%Fe=\dfrac{56}{72}\cdot100\%=77,78\%\)
\(\%O=100\%-77,78\%=22,22\%\)
b)\(M_{NH_3}=14+3=17đvC\)
\(\%N=\dfrac{14}{17}\cdot100\%=82,35\%\)
\(\%O=100\%-82,35\%=17,65\%\)
c)\(M_{N_2O_5}=14\cdot2+5\cdot16=108đvC\)
\(\%N=\dfrac{14\cdot2}{108}\cdot100\%=25,92\%\)
\(\%O=100-25,92\%=74,08\%\)
d)\(M_{CaCO_3}=40+12+3\cdot16=100đvC\)
\(\%Ca=\dfrac{40}{100}\cdot100\%=40\%\)
\(\%C=\dfrac{12}{100}\cdot100\%=12\%\)
\(\%O=100\%-\left(40\%+12\%\right)=48\%\)
Hai câu sau bạn làm tương tự nhé!