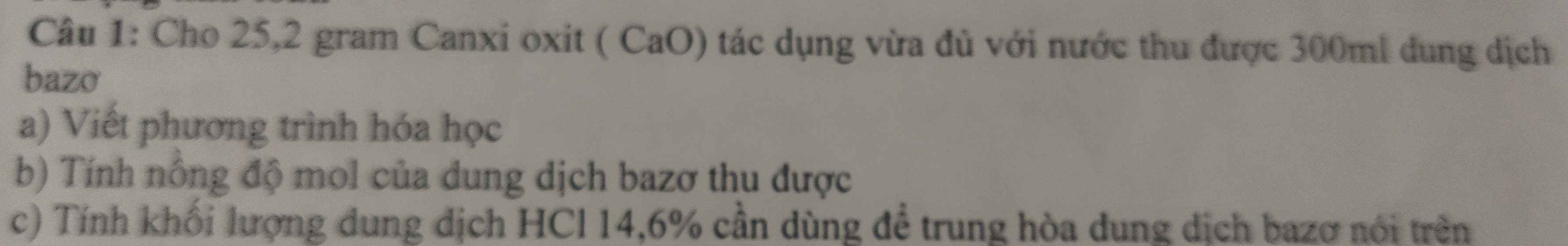Bài 4 :
\((1)4Al + 3O_2 \xrightarrow{t^o} 2Al_2O_3\\ (2)Al_2O_3 + 3H_2SO_4 \to Al_2(SO_4)_3 + 3H_2O\\ (3)Al_2(SO_4)_3 + 6KOH \to 2Al(OH)_3 + 3K_2SO_4\\ (4)2Al(OH)_3 \xrightarrow{t^o} Al_2O_3 + 3H_2O\\ (5)Al_2O_3 + 6HCl \to 2AlCl_3 + 3H_2O\\ (6)AlCl_3 + 3KOH \to Al(OH)_3 + 3KCl\\ (7)2Al(OH)_3 + 3H_2SO_4 \to Al_2(SO_4)_3 +6H_2O\\ (8)2Al + 6HCl \to 2AlCl_3 + 3H_2 (9) 2H_2 + O_2 \xrightarrow{t^o} 2H_2O\\ (10) Na_2O + H_2O \to 2NaOH\\ (11) 2NaOH + CO_2 \to Na_2CO_3 + H_2O\\ (12) Na_2CO_3 + 2HCl \to 2NaCl + CO_2 + H_2O\)
Bài 5 :
a) $Na_2O + H_2O \to 2NaOH$
$n_{Na_2O} = \dfrac{15,5}{62} = 0,25(mol) \Rightarrow n_{NaOH} = 2n_{Na_2O} = 0,5(mol)$
$\Rightarrow C_{M_{NaOH}} = \dfrac{0,5}{0,5} = 1M$
b) $2NaOH + H_2SO_4 \to Na_2SO_4 + 2H_2O$
$n_{H_2SO_4} = \dfrac{1}{2}n_{NaOH} = 0,25(mol)$
$\Rightarrow m_{dd\ H_2SO_4} =\dfrac{0,25.98}{20\%} = 122,5(gam)$
$\Rightarrow V_{dd\ H_2SO_4} = \dfrac{122,5}{1,14} = 107,46(ml)$