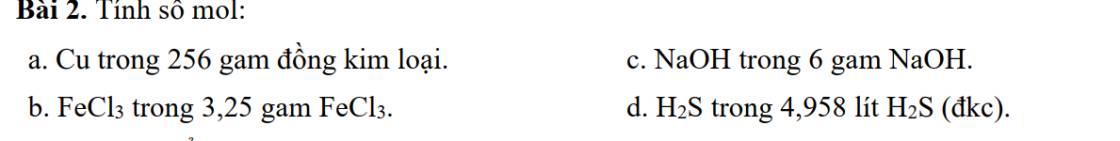\(a,n_{Cu}=\dfrac{m_{Cu}}{M_{Cu}}=\dfrac{256}{64}=4\left(mol\right)\\ b,n_{FeCl_3}=\dfrac{m_{FeCl_3}}{M_{FeCl_3}}=\dfrac{3,25}{162,5}=0,02\left(mol\right)\\ c,n_{NaOH}=\dfrac{m_{NaOH}}{m_{NaOH}}=\dfrac{6}{40}=0,15\left(mol\right)\\ d,n_{H_2S}=\dfrac{V_{H_2S\left(đkc\right)}}{24,79}=\dfrac{4,958}{24,79}=0,2\left(mol\right)\)
Đúng 3
Bình luận (1)
Các câu hỏi tương tự
Help mik với mấy bạn:Câu 14. Cho 13,5g Al phản ứng với dung dịch H2SO4 theo sơ đồ sau: Tính thể tích H2 thu được ở điều kiện chuẩn biết hiệu suất phản ứng là 90%Câu 15. Tính số nguyên tử Magnesium có trong 48 gam Magnesium Tính thể tích ở điều kiện chuẩn của 0,6022.1023 phân tử oxygen.Câu 16. Biết rằng tỉ khối của khí Y so với khí NO2 là 0,5 và tỉ khối của khí X so với khí Y là 2,5. Xác định khối lượng mol của khí X.
Đọc tiếp
Help mik với mấy bạn:
Câu 14. Cho 13,5g Al phản ứng với dung dịch H2SO4 theo sơ đồ sau:
Tính thể tích H2 thu được ở điều kiện chuẩn biết hiệu suất phản ứng là 90%
Câu 15. Tính số nguyên tử Magnesium có trong 48 gam Magnesium
Tính thể tích ở điều kiện chuẩn của 0,6022.1023 phân tử oxygen.
Câu 16. Biết rằng tỉ khối của khí Y so với khí NO2 là 0,5 và tỉ khối của khí X so với khí Y là 2,5. Xác định khối lượng mol của khí X.
bạn An đã thực hành xác khối lượng riêng của 1 viên sỏi , kết quả đo được khối lượng của viên sỏi là 70,2 g , thể tích viên sỏi là 27,3 cm^3 . Xác định độ chia nhỏ nhất của ống đong và tính khối lượng riêng của viên sỏi. help !!!
so sánh khối lượng và thẻ tích khí oxi ( ở ĐKTC) thu được khi phân hủy hoàn toàn 73,5g HClO3 hoặc 73,5g KMnO4
Vai trò của động cơ điện trong quạt điện?
A. Biến đổi điện năng thành cơ năng.
B. Biến đổi điện năng thành nhiệt năng.
C. Biến đổi điện năng thành quang năng.
D. Cả A và B.
Giải thích các hiện tượng liên quan đến sự truyền nhiệt của các chất rắn, lỏng, khí
Một vật làm bằng chì ở 30 oC, sau khi nhận thêm một nhiệt lượng là 15600J thì nhiệt độ của nó lên đến 130 oC. Hỏi vật đó có khối lượng là bao nhiêu? Biết nhiệt dung riêng của chì 130j/kg.k
Một ấm nhôm khối lượng 250g chứa 0,5 lít nước. Tính nhiệt lượng tối thiểu cần thiết để đun sôi nước, biết nhiệt độ ban đầu của nước và ấm là 30oC.
Một ấm nhôm khối lượng 250g chứa 0,5 lít nước. Tính nhiệt lượng tối thiểu cần thiết để đun sôi nước, biết nhiệt độ ban đầu của nước và ấm là 30oC
Thả một quả cầu nhôm khối lượng 4kg được đun nóng tới 130oC vào một cốc nước. Sau một thời gian nhiệt độ của quả cầu và của nước đều bằng 45oC. Tính nhiệt lượng mà quả cầu nhôm tỏa ra cho đến khi cân bằng nhiệt?