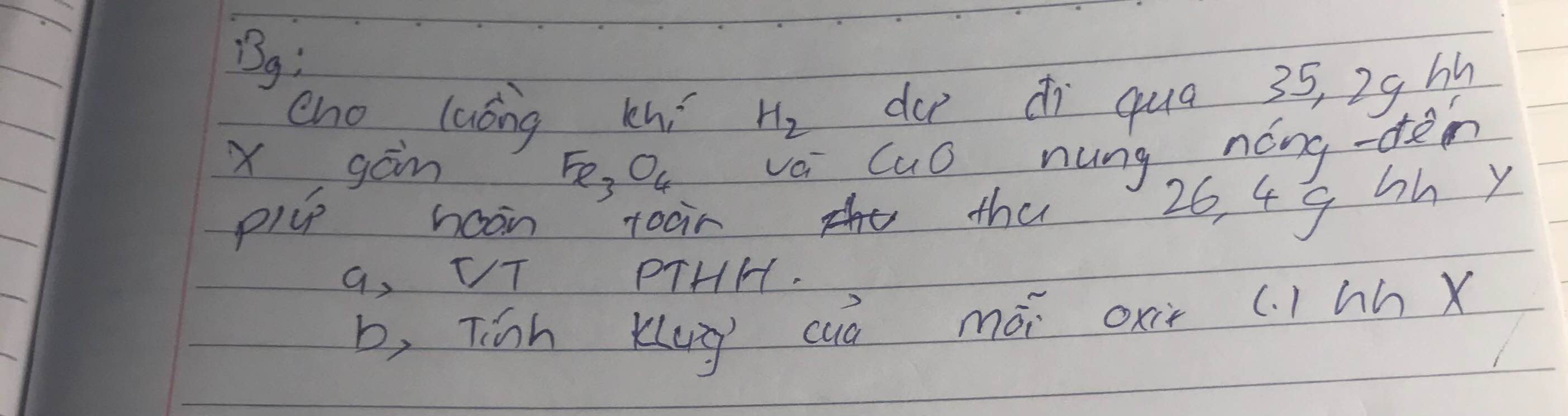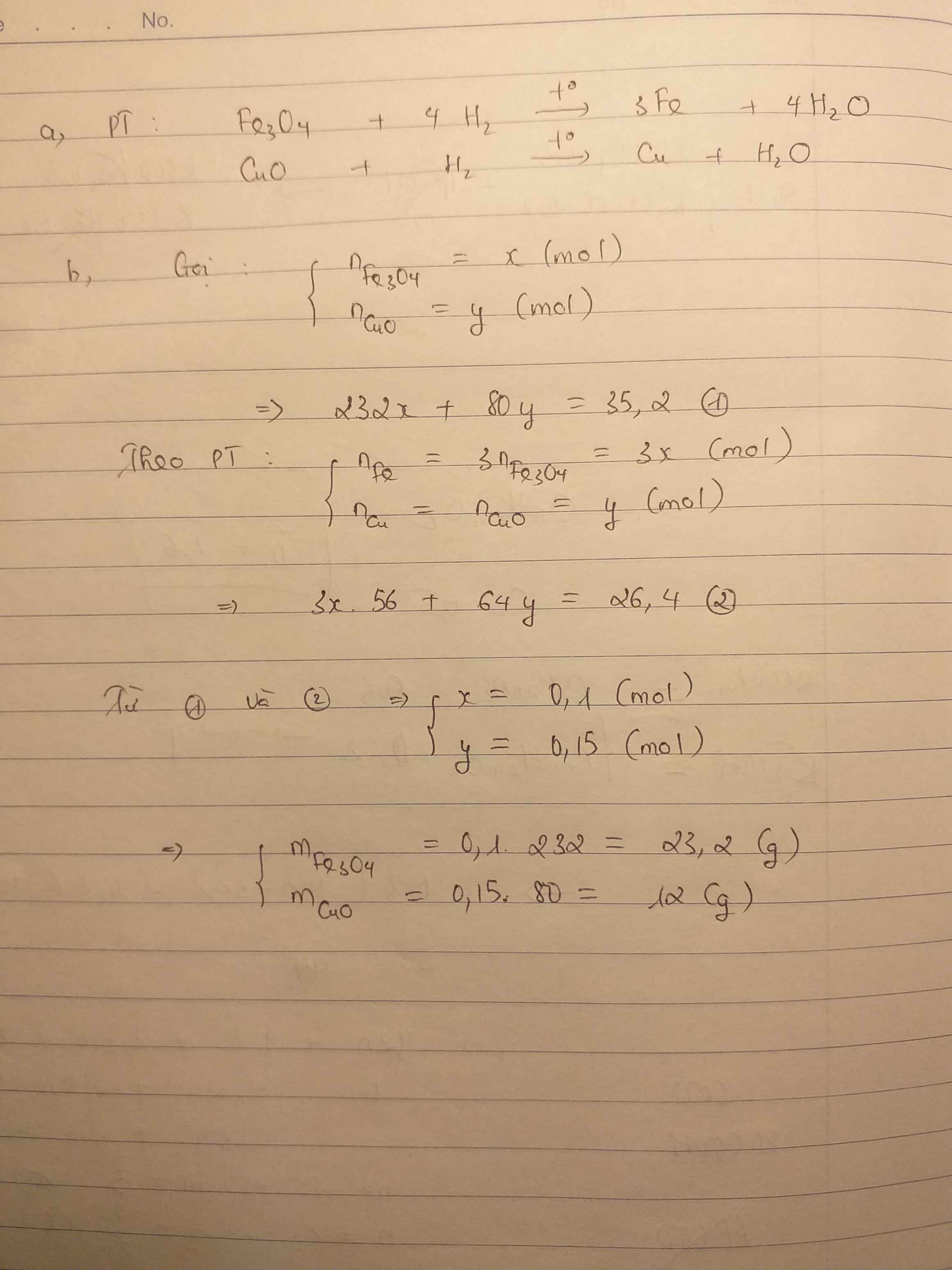a)
$Fe_3O_4 + 4H_2 \xrightarrow{t^o} 3Fe + 4H_2O$
$CuO + H_2 \xrightarrow{t^o} Cu + H_2O$
b)
Gọi $n_{Fe_3O_4} = a ; n_{CuO} = b$
Suy ra: $232a + 80b = 35,2(1)$
Theo PTHH :
$n_{Fe} = 3a ; n_{Cu} = b$
Suy ra: $3a.56 + 64b = 26,4(2)$
Từ (1)(2) suy ra a = 0,1 ; b = 0,15
$m_{Fe_3O_4} = 0,1.232 = 23,2(gam)$
$m_{CuO} = 0,15.80 = 12(gam)$