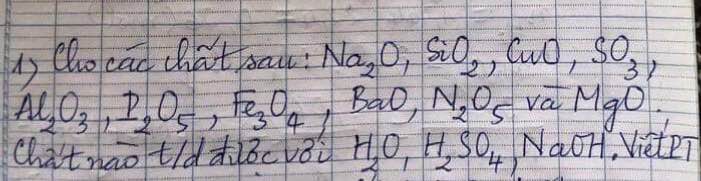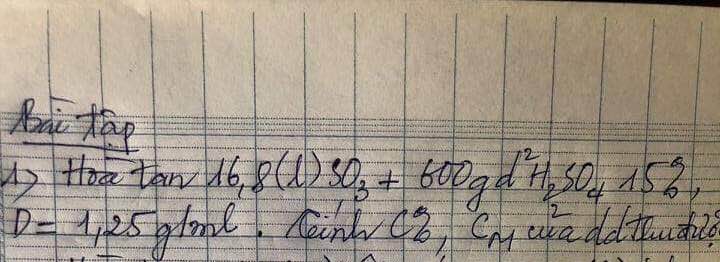a, PT: \(Mg+2HCl\rightarrow MgCl_2+H_2\)
\(MgO+2HCl\rightarrow MgCl_2+H_2O\)
Ta có: \(n_{H_2}=\dfrac{1,12}{22,4}=0,05\left(mol\right)\)
Theo PT: \(n_{Mg}=n_{H_2}=0,05\left(mol\right)\Rightarrow m_{Mg}=0,05.24=1,2\left(g\right)\)
\(\Rightarrow m_{MgO}=5,2-1,2=4\left(g\right)\) \(\Rightarrow n_{MgO}=\dfrac{4}{40}=0,1\left(mol\right)\)
b, Theo PT: \(n_{HCl}=2n_{Mg}+2n_{MgO}=0,3\left(mol\right)\)
\(\Rightarrow C_{M_{HCl}}=\dfrac{0,3}{0,08}=3,75\left(M\right)\)
c, Theo PT: \(n_{MgCl_2}=n_{Mg}+n_{MgO}=0,15\left(mol\right)\)
Ta có: m dd HCl = 1,05.80 = 84 (g)
⇒ m dd sau pư = 5,2 + 84 - 0,05.2 = 89,1 (g)
\(\Rightarrow C\%_{MgCl_2}=\dfrac{0,15.95}{89,1}.100\%\approx15,99\%\)