PTHH: 2KMnO4 \(\underrightarrow{t^o}\) K2MnO4 + MnO2 + O2\(\uparrow\)
\(n_{KMnO_4}=\dfrac{23,7}{158}=0,15mol\)
\(n_{O_2}=\dfrac{0,15}{2}=0,075mol\\ V_{O_2}=0,075.22,4=1,68l\)
\(\Rightarrow\) Đáp án A
Câu 28:
2Al + 6HCl \(\rightarrow\) 2AlCl3 + 3H2\(\uparrow\)
\(n_{H_2}=\dfrac{6,72}{22,4}=0,3mol\\ n_{Al}=\dfrac{0,3.2}{3}=0,2mol\\ m_{Al}=0,2.27=5,4g\)



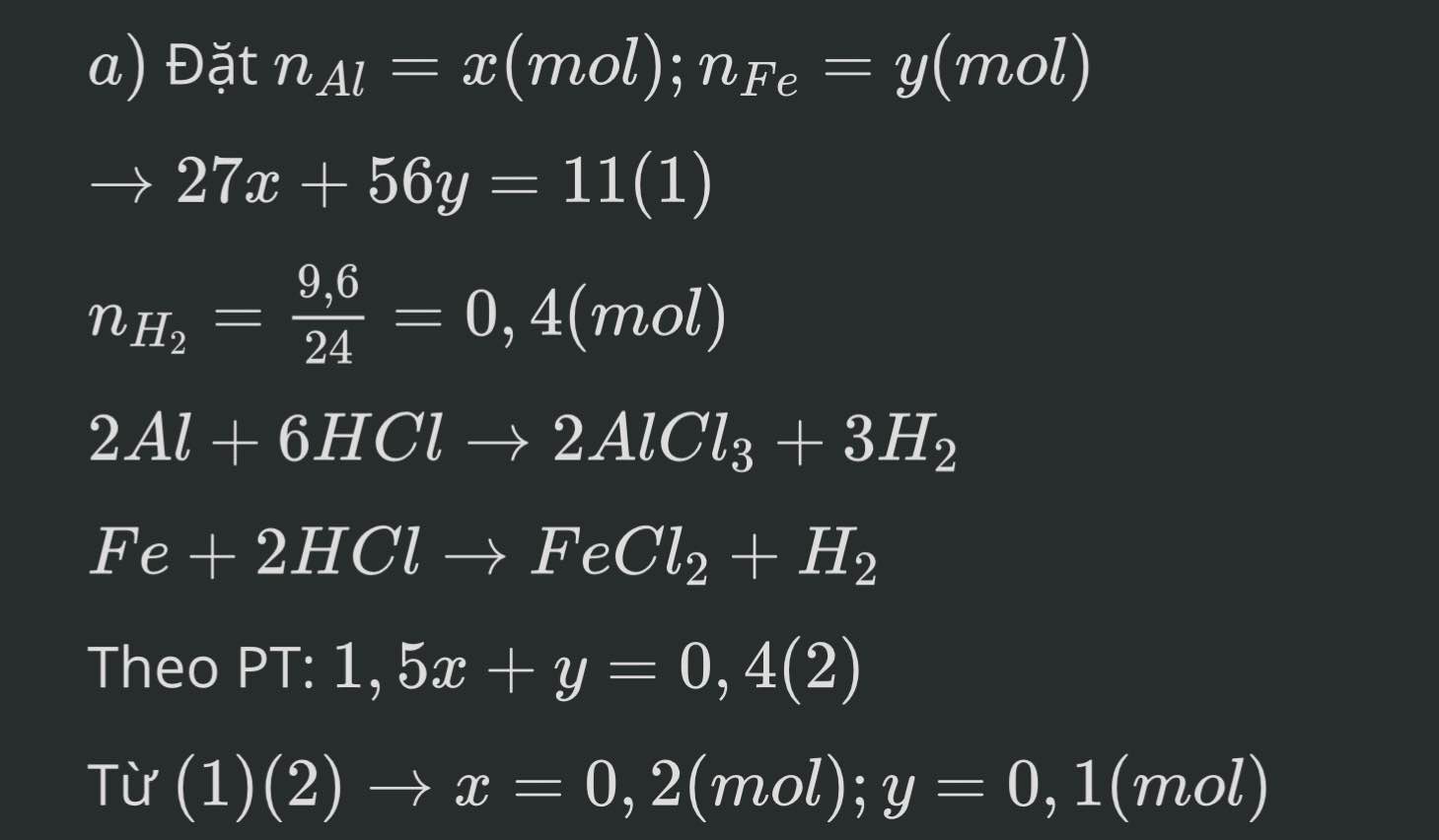


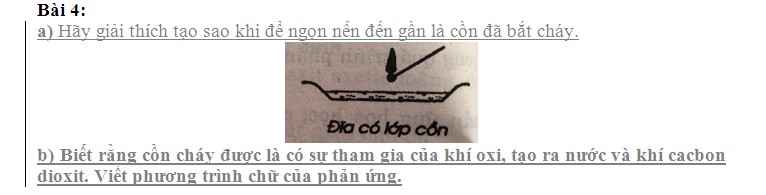 mấy anh chị giải chi tiết giùm em,em cần lời giải chi tiết ạ em xin cảm ơn
mấy anh chị giải chi tiết giùm em,em cần lời giải chi tiết ạ em xin cảm ơn
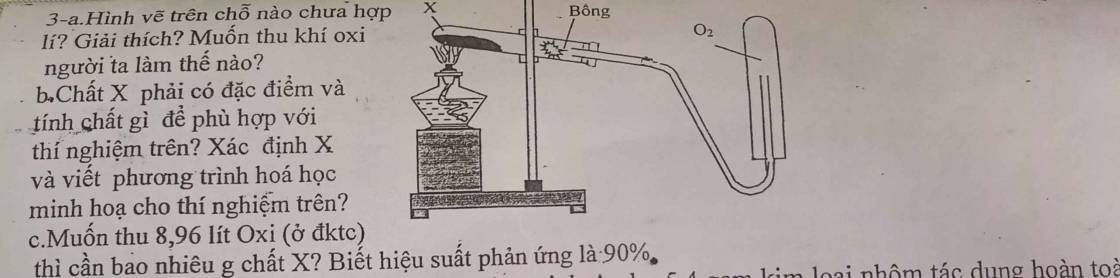 (em cần lời giải chi tiết về câu c ạ) em cảm onn
(em cần lời giải chi tiết về câu c ạ) em cảm onn