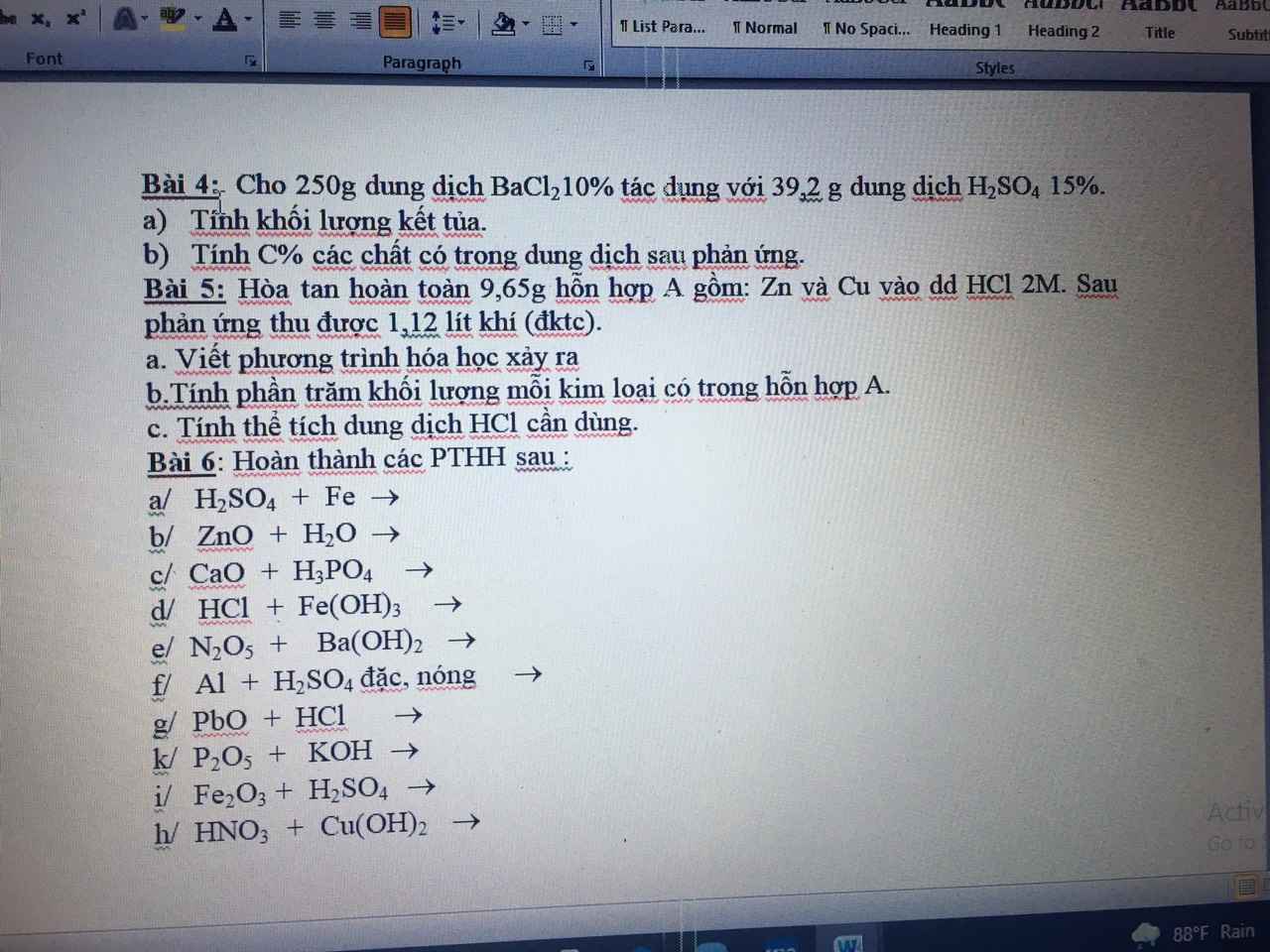
Bài 4:
\(a.n_{BaCl_2}=\dfrac{250.10\%}{208}=\dfrac{25}{208}\left(mol\right)\\ n_{H_2SO_4}=\dfrac{39,2.15\%}{98}=0,06\left(mol\right)\\ BaCl_2+H_2SO_4\rightarrow BaSO_4+2HCl\\ Vì:\dfrac{\dfrac{25}{208}}{1}>\dfrac{0,06}{1}\\ \Rightarrow BaCl_2dư\\ n_{BaSO_4}=n_{BaCl_2\left(p.ứ\right)}=n_{H_2SO_4}=0,06\left(mol\right)\\ m_{\downarrow}=0,06.233=13,98\left(g\right)\\ b.n_{HCl}=2.0,06=0,12\left(mol\right)\\ n_{BaCl_2\left(dư\right)}=\dfrac{25}{208}-0,06=\dfrac{313}{5200}\left(mol\right)\)
\(C\%_{ddBaCl_2\left(dư\right)}=\dfrac{\dfrac{313}{5200}.208}{250+39,2-13,98}.100\approx4,549\%\\ C\%_{ddHCl}=\dfrac{0,12.36,5}{250+39,2-13,98}.100\approx1,591\%\)