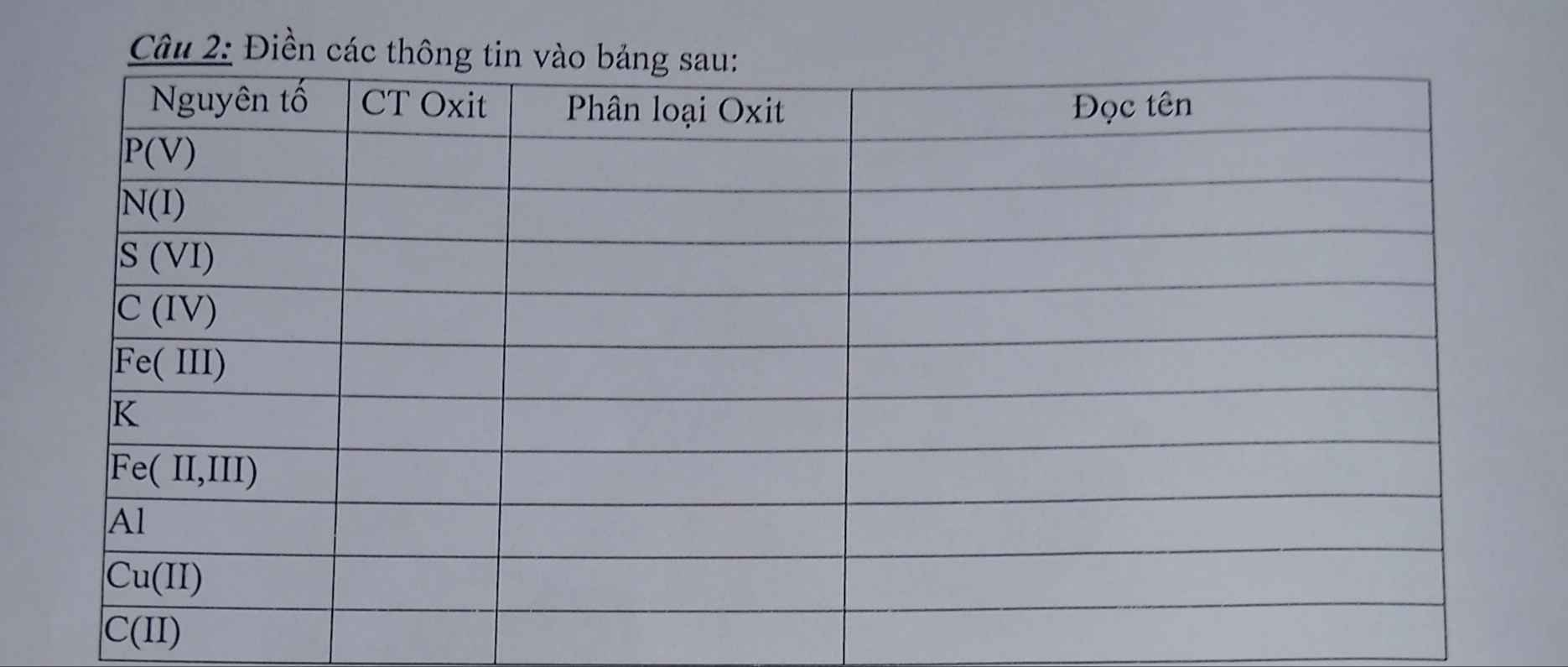
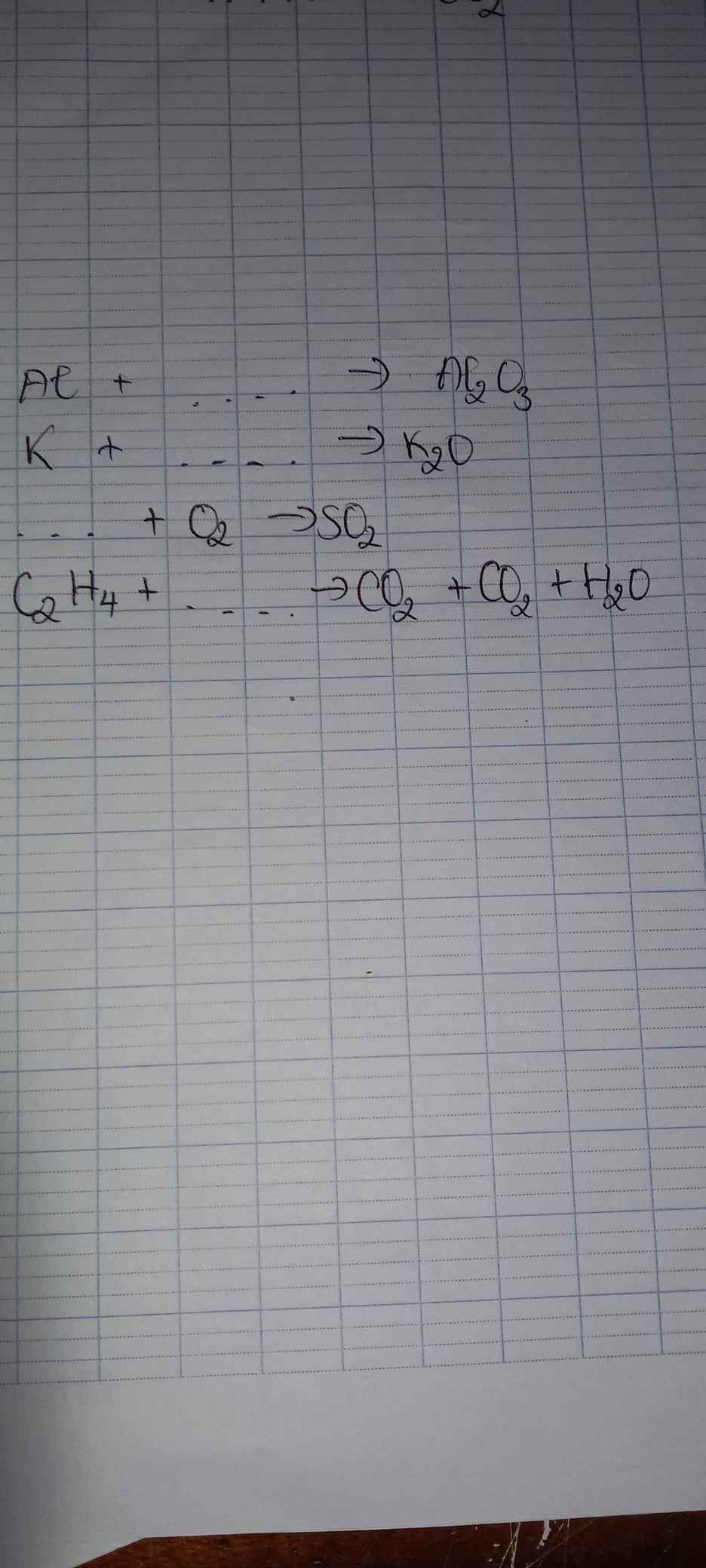a, PT: \(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
b, Ta có: \(n_{Al}=\dfrac{5,4}{27}=0,2\left(mol\right)\)
Theo PT: \(n_{Al_2O_3}=\dfrac{1}{2}n_{Al}=0,1\left(mol\right)\Rightarrow m_{Al_2O_3}=0,1.102=10,2\left(g\right)\)
c, Theo PT: \(n_{O_2}=\dfrac{3}{4}n_{Al}=0,15\left(mol\right)\Rightarrow V_{O_2}=0,15.22,4=3,36\left(l\right)\)
\(\Rightarrow V_{kk}=\dfrac{V_{O_2}}{20\%}=16,8\left(l\right)\)
d, PT: \(2KClO_3\underrightarrow{t^o}2KCl+3O_2\)
Theo PT: \(n_{KClO_3}=\dfrac{2}{3}n_{O_2}=0,1\left(mol\right)\Rightarrow m_{KClO_3}=0,1.122,5=12,25\left(g\right)\)
a) \(n_{Al}=\dfrac{5,4}{27}=0,2\left(mol\right)\)
PTHH: \(4Al+3O_2\xrightarrow[]{t^o}2Al_2O_3\)
0,2-->0,15--->0,1
b) \(m_{Al_2O_3}=0,1.102=10,2\left(g\right)\)
c) \(V_{kk}=\dfrac{0,15.22,4}{20\%}=16,8\left(l\right)\)
d) \(2KClO_3\xrightarrow[]{t^o}2KCl+3O_2\)
0,1<-----------------0,15
\(\Rightarrow m_{KClO_3}=0,1.122,5=12,25\left(g\right)\)