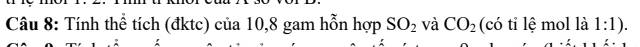Ta có: \(\dfrac{n_{SO_2}}{n_{CO_2}}=\dfrac{1}{1}\Rightarrow n_{SO_2}=n_{CO_2}\)
Đặt \(n_{CO_2}=n_{SO_2}=a\left(mol\right)\)
=> \(\left\{{}\begin{matrix}m_{CO_2}=44a\left(g\right)\\m_{SO_2}=64a\left(g\right)\end{matrix}\right.\)
=> \(44a+64a=10,8\)
=> \(a=0,1\left(mol\right)\)
=> \(\left\{{}\begin{matrix}n_{CO_2}=0,1\left(mol\right)\\n_{SO_2}=0,1\left(mol\right)\end{matrix}\right.\)
=> \(\left\{{}\begin{matrix}V_{CO_2}=0,1.22,4=2,24\left(l\right)\\V_{SO_2}=0,1.22,4=2,24\left(l\right)\end{matrix}\right.\)
=> \(V_{khí}=V_{CO_2}+V_{SO_2}=2,24+2,24=4,48\left(l\right)\)