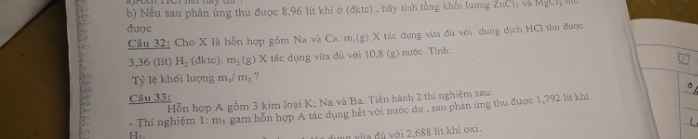Câu 32.
\(n_{H_2}=\dfrac{3,36}{22,4}=0,15mol\)
\(n_{H_2O}=\dfrac{10,8}{18}=0,6mol\)
\(2Na+2HCl\rightarrow2NaCl+H_2\)
x 0,5x
\(Ca+2HCl\rightarrow CaCl_2+H_2\)
y y
\(2Na+2H_2O\rightarrow2NaOH+H_2\)
x 2kx
\(Ca+2H_2O\rightarrow Ca\left(OH\right)_2+H_2\)
y ky
\(\Rightarrow\left\{{}\begin{matrix}0,5x+y=0,15\\kx+2ky=0,6\end{matrix}\right.\)
\(\Rightarrow\dfrac{0,5x+y}{kx+2ky}=\dfrac{0,15}{0,6}=\dfrac{1}{4}\Rightarrow k=2\Rightarrow\dfrac{m_1}{m_2}=\dfrac{1}{2}\)