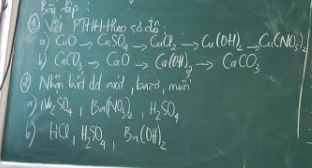\(6.a.Zn+2HCl\rightarrow ZnCl_2+H_2\\ n_{Zn}=\dfrac{19,5}{65}=0,3\left(mol\right)\\ n_{H_2}=n_{Zn}=0,3\left(mol\right)\\ \Rightarrow V_{H_2}=0,3.22,4=6,72\left(g\right)\\ b.3H_2+Fe_2O_3-^{t^o}\rightarrow2Fe+3H_2O\\ n_{Fe_2O_3}=\dfrac{19,2}{160}=0,12\left(mol\right)\\ LTL:\dfrac{0,3}{3}< \dfrac{0,12}{1}\Rightarrow Fe_2O_3dư\\ n_{Fe}=\dfrac{2}{3}n_{H_2}=0,2\left(mol\right)\\ \Rightarrow m_{Fe}=0,2.56=11,2\left(g\right)\)
\(4.Gốc-HCO_3khôngcóaxittươngứng\\H_2SO_4\\ H_3PO_4\\ H_2S\\HCl\\ HBr\\ HNO_3\\ H_2CO_3\\ H_2SO_3\)
\(5.a.CaCl_2:Muối\\ b.NaOH:Bazo\\ c.KNO_3:Muối\\ d.Ca_3\left(PO_4\right)_3:Muối\\ e.H_2SO_3:Axit\\ f.K_3PO_4:Muối\\ g.FeO:Oxitbazo\\ h.Ca\left(OH\right)_2:Bazo\\ i.Al_2\left(SO_4\right)_3:Muối\\ j.SO_3:Oxitaxit\\ k.Fe\left(NO_3\right)_3:Muối\)




 giải giúp mình câu 5 với ạ
giải giúp mình câu 5 với ạ