PTHH: 2Al + 6HCl --> 2AlCl3 + 3H2
Xét tỉ lệ: \(\dfrac{0,3}{2}>\dfrac{0,2}{6}\) => Al dư, HCl hết
PTHH: 2Al + 6HCl --> 2AlCl3 + 3H2
0,2---->\(\dfrac{0,4}{6}\)
=> \(m_{AlCl_3}=\dfrac{0,4}{6}.133,5=8,9\left(g\right)\)
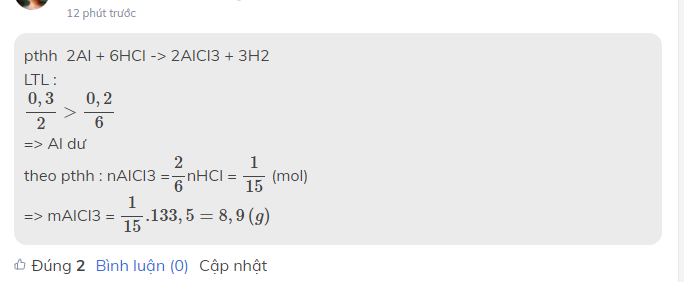
pthh 2Al + 6HCl -> 2AlCl3 + 3H2
LTL :
0,32>0,260,32>0,26
=> Al dư
theo pthh : nAlCl3 =2626nHCl = 115115 (mol)
=> mAlCl3 = 115.133,5=8,9(g)