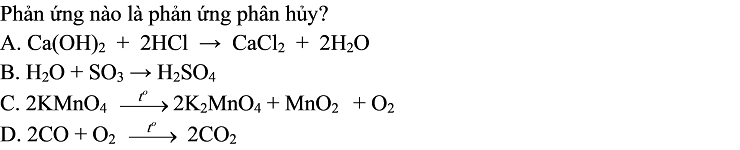Bài 3
\(n_{CO_2}=\dfrac{2,688}{22,4}=0,12mol\)
\(\left\{{}\begin{matrix}n_{Na_2CO_3}=x\left(mol\right)\\n_{CaCO_3}=y\left(mol\right)\end{matrix}\right.\)
\(Na_2CO_3+2HNO_3\rightarrow2NaNO_3+H_2O+CO_2\)
\(CaCO_3+2HNO_3\rightarrow Ca\left(NO_3\right)_2+H_2O+CO_2\)
\(\Rightarrow\left\{{}\begin{matrix}106x+100y=12,6\\x+y=n_{CO_2}=0,12\end{matrix}\right.\Rightarrow\left\{{}\begin{matrix}x=0,1\\y=0,02\end{matrix}\right.\)
\(\%m_{Na_2CO_3}=\dfrac{0,1\cdot106}{12,6}\cdot100\%=84,13\%\)
\(\%m_{CaCO_3}=100\%-84,13\%=15,87\%\)
\(\Sigma n_{HNO_3}=2\cdot0,1+2\cdot0,02=0,24mol\)
\(V_{HNO_3}=\dfrac{0,24}{0,5}=0,48l=480ml\)
Bài 4.
\(n_{HCl}=0,2\cdot2=0,4mol\)
\(n_{H_2}=\dfrac{3,36}{22,4}=0,15mol\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
0,1 0,3 0,15
\(MgO+2HCl\rightarrow MgCl_2+H_2O\)
\(\Sigma n_{HCl}=0,4\Rightarrow n_{HCl\left(MgO\right)}=0,4-0,3=0,1mol\)
\(\Rightarrow n_{MgO}=\dfrac{1}{2}n_{HCl}=0,05mol\)
\(m_{Al}=0,1\cdot27=2,7g\)
\(m_{MgO}=0,05\cdot40=2g\)
\(\%m_{Al}=\dfrac{2,7}{2,7+2}\cdot100\%=57,45\%\)
\(\%m_{MgO}=100\%-57,45\%=42,55\%\)